Sunday, 17 August 2008
Well we've arrived safely in Key Largo for another season of field work, only to encounter a potential hurricane headed our way. As I write this tropical storm Fay is bearing down upon us. It is currently somewhere over Cuba & is predicted to pass by Florida tomorrow morning. There has been a partial evacuation of some of the keys, but we will be sticking it out in our field house unless it gets really bad. The most recent models show it missing us to the west so hopefully all will be well after a bit of stormy weather tomorrow.
We will be delayed in our research by a day however, as NOAA has instructed us to pull our boat from the water until the weather clears. We have all our equipment set up and ready to go for our planned experiments, so we are hopeful that the storm will pass without incident and we will get a good spawn this year, providing an abundance of larvae to work with.
This year's experiment involves rearing coral larvae from multiple different parents at three separate temperatures (cool, average, and hot). Our aim is to better understand the effect of changing ocean temperatures on coral larval development, and eventually, how changing temperatures affect the expression of specific coral genes. This experiment will be carried out in parallel in Puerto Rico where Iliana is working with the team from SECORE.
So, please check back to follow our progress through the season & we'll try to post updates as often as possible.
-NP
Monday, 18 August 2008
This morning we awoke to the sound of rain on our windows, and promptly got out of bed to go take apart and protect all of the delicate parts of our larvae rearing system in preparation for the big storm. It was a bit frustrating having to dismantle the system that we had spent all weekend building, but if it were to flood and damage our equipment it would be far worse!
We spent the rest of the morning repairing old spawn collecting nets under the shelter of the back porch. The serious weather didn't start until about mid day, when we started to see some strong windsand heavy rain. The water in the canal was at the uppermost edge of the concrete during high tide, but fortunately we avoided any flooding. The rest of the day was spent indoors where we took some well needed time to plan out our experiment in a bit more detail and work out any minor problems.
Much of the complication in this experiment involves the fact that we are attempting to generate crosses from specific individual coral colonies out on the reef that we already have genetic "fingerprints" for. By crossing these individuals with each other and investigating how well their offspring fare in different temperatures we will be able to get a sense for how larval performance is related to heritable effects. Experiments such as this are common in plant and animal breeding research, but become much more difficult when working with wild critters. The success of our research depends in a large part on being able to collect gametes from a number of parent colonies of known genotypes for our crosses. If we miss the spawn because of the weather we would have to delay everything for another year, and if the corals are badly damaged by the storm they may spawn early. Hopefully this is not the case.
The worst of the storm is now already past us. Tomorrow will continue to be windy and rainy however, and it is unlikely that we will be able to go out to the reef since the waves are forecasted to be between 4 to 7 feet. So we have one more day of waiting ahead of us before we can get out on the reef.
-NP
Tuesday, 19 August 2008
We were teased with a few brief relapses of violent downpours, but Tropical Storm Fay has definitely moved on. Still-strong winds out on the ocean kept us off of the water and are forecasted to keep us from being able to collect any spawn tomorrow night as well; for making preparations outside on land, though, I personally welcome the cooling wind and am sure I’ll miss it when it’s gone.
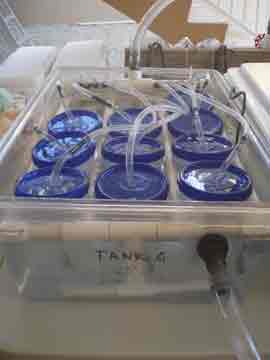
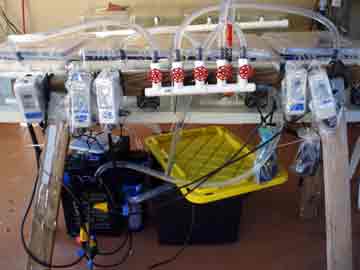
We spent the day re-assembling the experimental set-up and organizing the myriad of gamete receptacles that the gathering, separating, and crossing of egg and sperm will require. Nick installed the chillers and temperature controllers for each of the dozen tanks (except one of the elusive chillers, still in transit, hopefully to arrive tomorrow) and I connected the long fingers of tubing to each of the ten kreisels in each tank. We filled up each of the three systems (four tanks per high, average, and low temperature treatment) with ocean water. The first time the entire set-up had been tested, it was very rewarding to hear the happy hum and gurgle of the pumps and watch the bubbles play follow-the-leader from tank to tank.
-KG
Wednesday, 20 August 2008
It's 8:30 and I'm sitting in the house writing the day's blog entry, to my great disapointment. We should be out on the reef right now preparing to collect coral gametes bundles as they are released en masse by thousands of tiny coral polyps, but the weather is still working against us.
We put the boat back into the water earlier today after shoveling off the 3 plus feet of sea grass & sargassum that had accumulated on the boat launch after the storm. After a stop for fuel we headed out to the reef to check out the conditions to see if a night dive would be feasible. About halfway out to the reef & totally drenched by the 3-6 ft waves along the way, we realized that a dive would not be possible. After three days of sustained winds at above 20 knots we were very concerned about the corals though, so Dana & Abel donned their snorkeling gear and went out to check on them. Similar conditions during hurricane Dennis reduced many large branching corals to rubble, with entire branches broken off at the base. So we wanted to see if the corals in our study plots had been badly damaged by the rough conditions. Katey & I remained on the boat as lookouts to keep an eye on the snorkelers as they bobbed around in the rolling waves. Since it was too rough to connect the boat to the mooring buoy, we drifted nearby until they gave the signal to come pick them up.
After we picked them up it was clear that there would be no diving this evening. In addition to the wicked waves there was a strong current and very low visibility from all the sediment kicked up from the storm. The good news was that the corals appeared to have suffered very little damage. Their elegant branching form belies the toughness of their calcium carbonate skeleton. Except for one colony that had gotten a thick rope from a commercial lobster trap tangled all around it, everybody looked pretty good.
The poor colony with the heavy rope tangled in its branches was another story. The delicate tips where the most rapid growth occurs were broken off all over the place and some rather substantial chunks had cracked off as well. Frustratingly, the snorkelers could do nothing about it as it is a crime, with significant penalties, for anyone other than the owner to move a commercial lobster trap from where it is found. Even if that happens to be horribly tangled around a threatened species...
We will try to go out again tomorrow. We will have two boats at our disposal then, and will hopefully be able to cover both sites where we would like to collect spawn. It is now the fourth day after the August full moon. Acropora palmata typically spawn on the third to fifth night after the moon around here, so with luck we may still be able to get the gametes that we need to conduct our experiment tomorrow.
-NP
Thursday, 21 August 2008
Mixed news today. Iliana and the folks from SECORE were able to collect plenty of spawn last night in Puerto Rico. You can read the details of their success on the SECORE blog. This is great news & Iliana has begun the temperature stress treatment we have been planning for so long.
Things are less exciting here in Florida. It was windy again today and worse out on the water. With 3-6 foot waves and extremely poor visibility we decided that going out for a night dive was still not a good idea. This conclusion was supported by the folks from NURC who had gone out earlier to the Aquarius underwater habitat and said the visibility was so poor that they had trouble finding it...If they can't find a 50ft yellow submarine habitat out there, I don't think we have a chance of finding our little coral friends.
We will try one more time to collect A. palmata gametes tomorrow night if conditions improve. Failing that, we may shift our focus to another species Montastrea faveoata, the Mountainous Star Coral. This species is relatively common around the Florida Keys, is much more consistent in its spawning habits and will allow us to address some of the same questions regarding heritability and resilience to thermal stress.
....
I had thought that I could work the discussion of the experiment we have been planning to conduct into the daily postings more subtly, but as it seems we may not get the chance to actually do what we had planned I'll give you all a little more insight into our plan here.
Increasing sea surface temperature due to global climate change is one of the major stresses facing coral populations worldwide. Understanding how corals deal with thermal stress on a genetic level will help us conserve coral populations by enabling us to target conservation efforts based on genetic profiles, and potentially aid in breeding or transplanting efforts. Our research is one of the first attempts to learn about what genes are involved in the coral animal's response to temperature stress. Our plan to accomplish this involves raising coral larvae at three temperature treatments; 77, 80, & 86 degrees Fahrenheit. This will be done in a series of aquaria attached to temperature controllers that keep the water within 1 degree of the target temperature. Inside each tank there is a series of small cups called kreisels that keep the tiny larvae in, but allow water to exchange carrying out wastes and bringing fresh water to the coral babies.
We prepared in advance several thousand test tubes with various preservatives in which we will collect samples of the larvae as they develop. By looking at these samples under the microscope back in the lab we can observe how the various temperatures impact larval development. The theory being that warmer waters cause the corals to develop faster. Additionally, we will save larvae to extract RNA from. By looking at the RNA we can see which genes are actually being expressed by the coral. Using something called a microarray, we will be able to compare which genes are used by the corals at high temperatures and compare that with those that are used by the corals at lower temperatures.
From a geneticists perspective it is important that all of this work be done with coral larvae rather than adult corals. This is because as the larvae develop into adults they take on specific types of symbiotic algae called zooxanthellae, that live within their tissues and supply the coral with food produced through photosynthesis. Adult coral tissues contain both plant and animal cells; from the coral itself and from the zooxanthellae algae, so DNA extractions from adults will consist of genes from both. This makes it difficult (often impossible) to tell which genes came from which source. To avoid this problem we make our annual attempt to gather the symbiont free larvae during the few nights a year when the corals spawn.
-NP
Friday, 22 August 2008
The corals spawned!
We headed out for our evening dive at Elbow reef, with little hope of collecting any spawn from the elkhorn coral. A scouting team had gone out earlier to check the water condidtions and the wind was still blowing up substantial waves. We had access to a larger vessel tonight thanks to NURC and captian Tim, so we figured we could give it a shot and hopefully come back with Montastrea spawn even if we missed Acropora.
Hopes were not high since it was already the 6th night after the full moon, and Acropora has not been observed to spawn that late here in the Keys. The colonies on Elbow reef tend to go later than others in the region so it offered the last hope of collecting spawn from this species.
We got into the water at about 7 to check on the corals and set up the marker bouys that we use to locate specific colonies in the dark. In general the corals looked good. A few broken branches here and there evinced the passing of the recent storm and another poor colony was wrapped in a tangle of line fron another errant lobster trap bouy.
After marking the colonies with the unique genetic backgrounds that we were interested in with colored foam bouys and glowsticks, we returned to the boat to wait for dark. At about 10PM we returned to the water to see if the coral polyps had begun to set their little pink bundles of gametes.
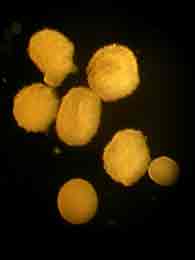
The species we are working with are hermaphrodites, each individual polyp producing both sperm and eggs. The gametes are packed in to tiny little bundles that are released from the polyp's multi purpose orafice (mouth/anus) once a year. The bundles are positilvely bouyant, so they congregate at the surface of the water where they break apart into sperm and eggs and each goes their separate ways to find a suitable cell to fertilize.
Tonight we captured gametes from three colonies. Two of them were probably the last remnants of spawning that had occured a day or two before, but the third gave us well over a half a liter of bundles! Asynchronous timing of gamete release could well lead to the extinction of a lineage since it will never be able to mate with other nearby colonies. Crossing work in the lab shows that the coral gametes do not stay viable in the water for long. If fertilization doesn't occur within a few hours the corals have to wait a whole year for another chance. And if they are consistently late or early they will never mate successfully.
Back on the boat on the trip home we worked frantically to prepare dilutions from all the colonies and set up the desired crosses. We let the sperm and egg soutions incubate for about an hour than rinsed off the excess sperm. After taking a few samples for later analysis, we divy'd up the fertilized eggs into the kreisels in our temperature treatment tanks.
By the time this was all finished it was around 4AM. We get about 4 hours of sleep then have to be up for the next round ouf sampling & hopefully more diving and spawn collecting tomorrow...
-NP
....
Coral research is all about teamwork. I mean, you dive in buddy teams; cooperation is literally mandated by law. Though I’m kind of a third-party participant in the proceedings out on the dive boat, I really enjoyed seeing how so many people- and pieces of equipment- could execute such a complicated sequence of movements amidst constantly changing and unpredictable conditions to achieve a common goal.
Though an afternoon scouting mission still had everyone concerned about the conditions, tonight was the last night in the spawning window for Acropora; it was pretty much now or never for this species (or at least until next year). There were numerous contingency plans, but thankfully, the sea was (compared to everything I’ve seen) calm, and some colonies spawned!
Margaret and I quickly pipetted the layer of bundles off of the water- and the feasting predatory plankton- to measure the volume of bundles and dilute them so that when opened to release their bounty, the ratio of sperm and eggs would be optimal. As we worked on Orange, Nick brought up small collections came in from three other colonies, and we set to work mixing some batches of gametes. (I have found that not many scientists feel confident doing dilutions without a calculator, even when sitting at a lab bench in a sterile laboratory. I think that Nick and Margaret deserve some acknowledgement for doing them while bobbing on the ocean, kneeling in front of an actual bench after just being 20 feet under the ocean for an hour and a half. Hats off to you!)
-KG
Sunday, 24 August 2008
The corals spawned some more!
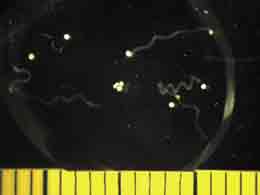
We now have multiple batches of coral larvae going in the temperature tanks. These require continuous monitoring and sampling every 8 hours. Between that and setting up the crosses I am able to get no more than 3 hours sleep in random intervals through the day. Things are very busy & blog postings are falling behind. Hopefully things will settle down soon, but it is very exciting to have all these larvae thriving! Some are even starting to swim already.
More details to come...
-NP
Monday, 25 August 2008
It has been very hectic around here the past few days with diving, sampling, and caring for all of our baby corals all happening at once. Last night a crew went out to the reef to try collecting more Montasrea spawn, but only saw a very slight trickle of bundles. It appears that the spawning may be over. Using the gametes that we collected on previous nights we were able to generate three batch cultures for our temperature experiments. Only one of the cultures is from Acropora. The other two are Montastrea. This will give us some interesting cross species comparisons, and the large Montastrea culture should be suitable for the complete microarray analysis we have planned.
In the temperature tanks the coral larvae have been doing well with clear differences in their development. After making the crosses by combining equal amounts of gametes from each of four different parents and letting them fertilize, the zygotic larvae were divided among replicated temperature tanks. About 24 hours after ferilization the breakdown of all the unfertilized eggs occurred, leading to a slight panic & the need to clean the kreisels manually to maintain the high water quality that the larvae need. Since then things have looked really good and healthy. It is clear though that the larvae in the high temperature treatment are developing faster since they were the first to start swimming, however they are also smaller in size than the larvae raised at lower temperatures and they suffered greater mortality during the crash at 24 hours post fertilization. Ironically the temperature of our high temperature treatment is 30 degrees C (about 86 F). This is about what the water temperature is here in the waters around the Keys. Our preliminary observations indicate that the larvae raised at this high temperature, though developing rapidly are performing less well. This does note bode well for the corals reproducing here in Florida.
Our night time collection dives are over at this point. We went out today to collect some tissue samples from adult parent corals that we need genetic information from. And tomorrow we plan on participating in a survey of the area reefs for storm damage, to identify fragments of Acropora the may have been broken off in the storm. Keeping track of broken fragments is important becaust this represents the primary mode of reproduction in Elkhorn corals around here. Asexual reproduction occurs whenever a chunk of an adult colony is broken off of the main colony and continues to grow separately. Here in Florida where sexual reproduction is relatively rare, fragmentation is critical to maintaining population size. This is a good thing in that it increases the number of coral colonies that are present in the local ecosystem, but continued asexual reproduction leads to reduced genetic diversity in a population and limits ability to adapt to changing conditions.
-NP
Tuesday, 26 August 2008
The last few days have been pretty busy- hence, the need for a retrospective entry.
On Tuesday, Nick and I accompanied Dana and Abel on aportion of Dana’s Acropora-monitoring circuit. Nick and Abel dove, and Dana and I snorkeled at a variety of different reefs. A newcomer to the reef ecosystem, I was thrilled with my first swim amid a school of sergeant major damselfish, and my first view of the species that I came to Florida to study.
-KG
Wednesday, 27 August 2008
Most of what Nick and I have been up to is sampling. Three times a day (8am, 4pm, and midnight), we suck up droplets of larvae from each kreisel and drip them into different kinds of preservative, so that they can be analyzed later for development and gene expression. This may not sound too difficult or time consuming, but as we remove larvae, as larvae die, and as the larvae start swimming throughout the water column rather than swirling like pick-a-ducks on the surface, it takes more time (and more patience) to select sufficient numbers for each vial. Yesterday, we switched to sampling once a day as the differences between time points becomes less significant as the larvae develop.
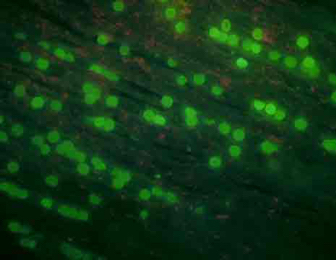
We also started photographing subsets of the larvae. These glamour shots are taken by a camera, mounted atop the dissecting microscope, set so that the shutter remains open for 5 seconds. The movement of the larvae appears as a snapshot of neon-green ribbon dancers. With the proper software, the distance traveled can be measured and used to calculate swimming speed.
-KG
Final Update, 2008
Apologies for the wait, but busy schedules of sampling, diving and travel, along with some issues with the web server have delayed the posting of our final entries.
After almost a full week of caring for our young larvae the populations suffered a substantial crash on about day 6. While there was no clear cause, we suspect that it may be due to some innate developmental problem with the larvae, as all populations in all three tank systems, as well as the batch cultures being raised by Margaret Miller's group, all experienced a drop in numbers at about the same time. This has left us with fewer settlers than we had originally anticipated, but fortunately it occurred late enough in the developmental cycle to allow us to collect the samples we needed for our development and gene expression research.
Our final day here in Florida was spent breaking down the aquarium system and packing up all our field gear to ship back to Pennsylvania. It was truly unbelievable the amount of equipment we had to pack and ship. Katey and I high tailed it back north just as the bad weather from Gustav and Hannah were about to start double teaming south Florida. Katey began her first semester as a grad student at Penn State while I headed over to Columbus Ohio to assist with counts of settled Acropora palmata larvae collected by SECORE in Puerto Rico.
In the coming months we will continue to work with our samples; analyzing them under the microscope for developmental differences, and begin the process of extracting RNA and sequencing the expressed genes for the microarray development.
Thanks for reading along & wish us luck.
Til next year!
-NP